 Copyright Independent Balkan News Agency, 2021
https://www.pioneerindustrial.com/wp-content/uploads/2021/11/Biogas-Plant.jpeg
1048
2000
Karrie Williams
https://www.pioneerindustrial.com/wp-content/uploads/2020/12/Pioneer-Logo-Color-min.png
Karrie Williams2021-11-17 21:25:392021-11-17 21:25:44Waste Burner Systems
Copyright Independent Balkan News Agency, 2021
https://www.pioneerindustrial.com/wp-content/uploads/2021/11/Biogas-Plant.jpeg
1048
2000
Karrie Williams
https://www.pioneerindustrial.com/wp-content/uploads/2020/12/Pioneer-Logo-Color-min.png
Karrie Williams2021-11-17 21:25:392021-11-17 21:25:44Waste Burner Systems
The oil and gas industry experiences a high degree of pushback these days. Tighter regulations and environmental activism affect the productivity of the industry. Increasing difficulty in extracting hard-to-reach oil, gases, and scarce resources poses a tough challenge. These factors make it difficult for an oil and gas operation to be successful and remain profitable.
Many would say that this is inevitable and that we should move away from the industry going forward. While there is room for these discussions, the impact of oil still warrants recognition. A great majority of our modern-day technologies would not exist without this diverse industry.
Our society is now beyond the imagination of those who lived just three or four decades ago—the lives we live now are reminiscent of the great science fiction novels of the 1950s. The source of these technological advances is certainly vast and complex. Still, many of our advancements would have been impossible without the harnessing of oil and gas.
Today we will explore a significant, impactful part of the oil and gas industry: oil refining and its resulting products. You may be thinking that refined oil is just something we put into our gas-guzzling cars, commuting to work and back. This, however, is not the case at all. The colors you see around you, the products we use to eliminate body odor, toys, toothpaste, or the computer or phone you’re reading this on…
These, and thousands more, were likely produced from an oil-derived product at some point.
A Crude Explanation of Oil Refining
Most oil needs to be refined to have any usefulness. Why is that? Well, oil out of the ground is referred to as crude oil: a variable mix of hydrocarbons. Crude oil is extracted from underground via a well or from operations like oil sand mining. At this stage, the product is dense and unfit for practical use. However, it can now be refined into several different components, all serving their own purpose.
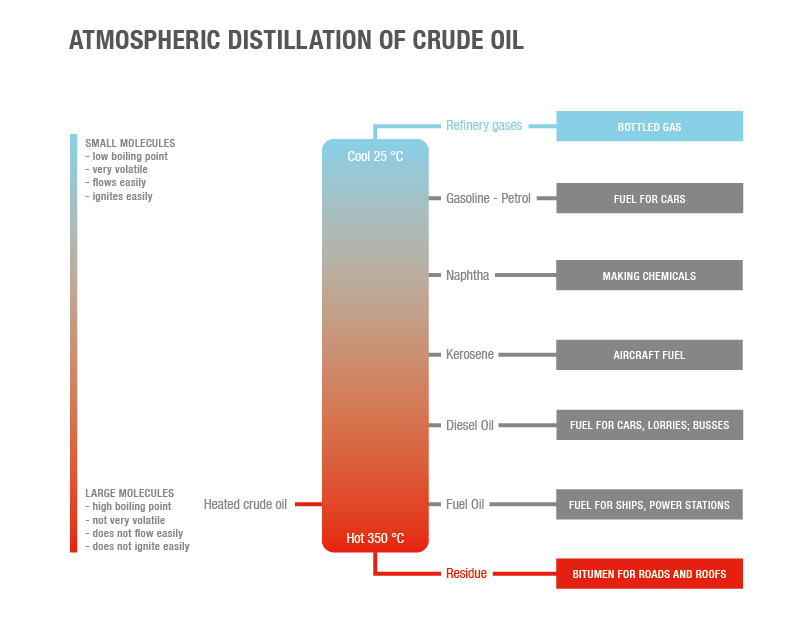
Looking at an oil refinery, you’ll notice tall, cylindrical towers. These towers are called distillation columns. Similar to simple water distillation, these columns heat up crude oil and separate the vapors into their individual components. The height of these columns is important: it aids in inducing these separations. Within the columns, trays are seated at various stages. These trays have specially designed pores, which allow vapors to flow up and through.
The image above shows how the temperature gradient of a distillation column operates. At different heights and temperatures, the crude oil vapors begin to condense. A “lighter” fluid has a lower boiling point, so its vapors will travel higher up the column before condensing. This condensation then forms liquid on the tray surface area and is drawn off.
As an example of this process, let’s take a look at diesel and gasoline. Gasoline has a lower boiling point than diesel—gasoline is lighter. Both are refined from the crude oil that enters the column and is superheated. The temperature decreases as they rise in the column, and the diesel (having a higher dew point) condenses and is extracted first. Gasoline vapors will rise even higher before it condenses and is extracted separately.
Now, from one input of crude oil, you have both gasoline, diesel, and other products. After this, there are likely further refining steps to be taken. Gasoline, for example, undergoes catalytic cracking to further break up the hydrocarbon chains. It may also be sent through alkylation. This process lengthens hydrocarbon chains and creates blending stock for premium gasoline products. What isn’t refined into gasoline, fuel, or distillate still has a long road ahead of it, as we will see below.
By-Product by By-Product
Distilling crude oil results in a variety of chemical by-products. Some of these are extremely useful, and they are sold to chemical companies for manufacturing purposes like creating plastics.
Take polypropylene for example. It is first derived from distilling crude oil into fractions. It can then be polymerized, which creates an extremely robust manufacturing plastic. Propylene is used in the manufacturing of many industrial and household items. It is strong, chemical resistant, and malleable. Next time you visit your neighborhood park, take a look at the big slides. You can bet that it’s made of polypropylene (or a related polymer).
Now take a moment to think of the last time you refinished a room and gave it a fresh coat of paint. That paint wouldn’t have been as easy to use, readily available, or cost-effective without oil and gas. Or picture the interior of your car. The plastics that give it structural stability, and a lower price tag, are from the vastly versatile polymers refined from oil.
The list of oil and gas by-products goes on and on. A post over at Ranken Energy gives a diverse sample of 144 items, but they estimate the number of by-products to be around 6000!
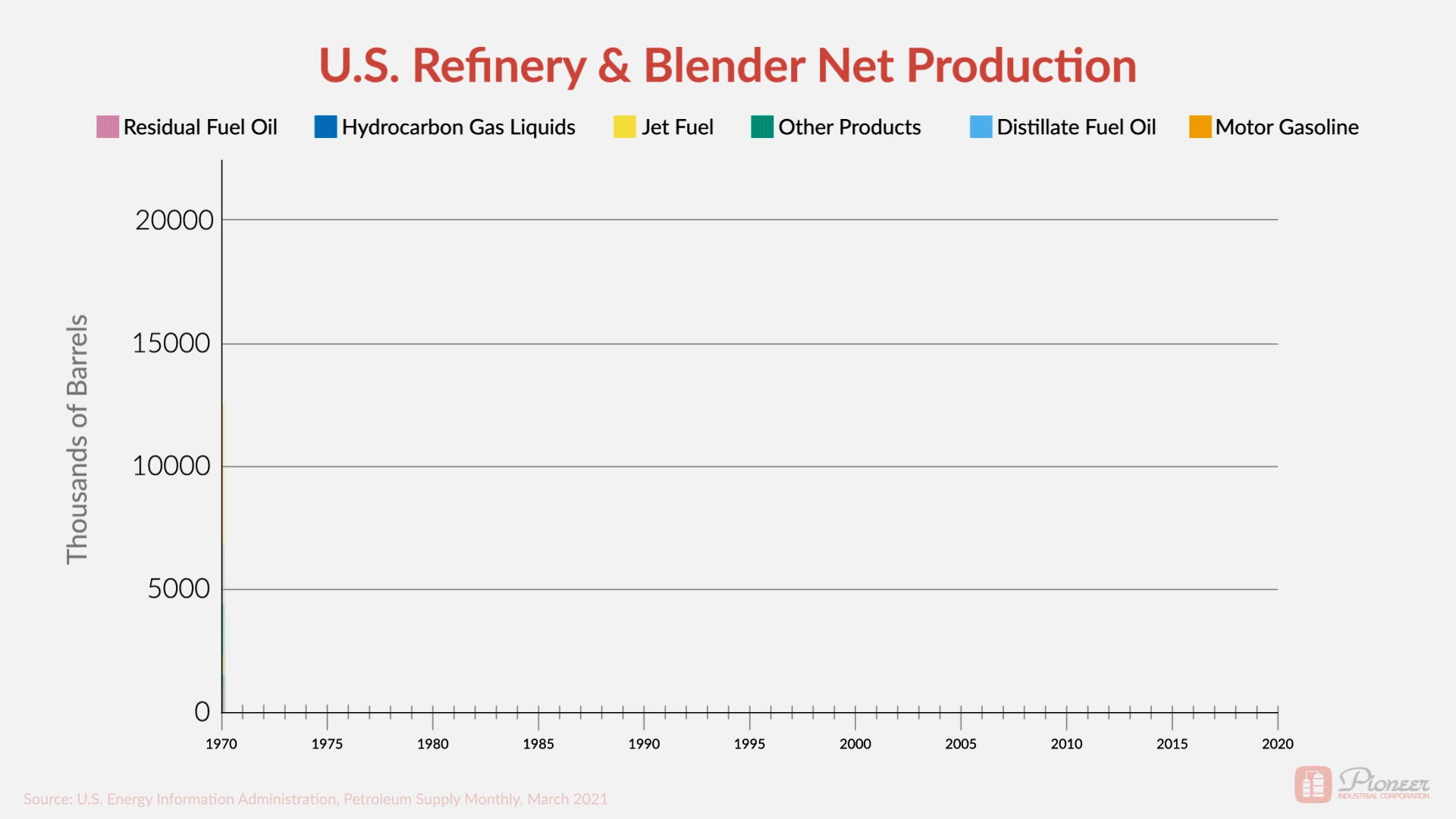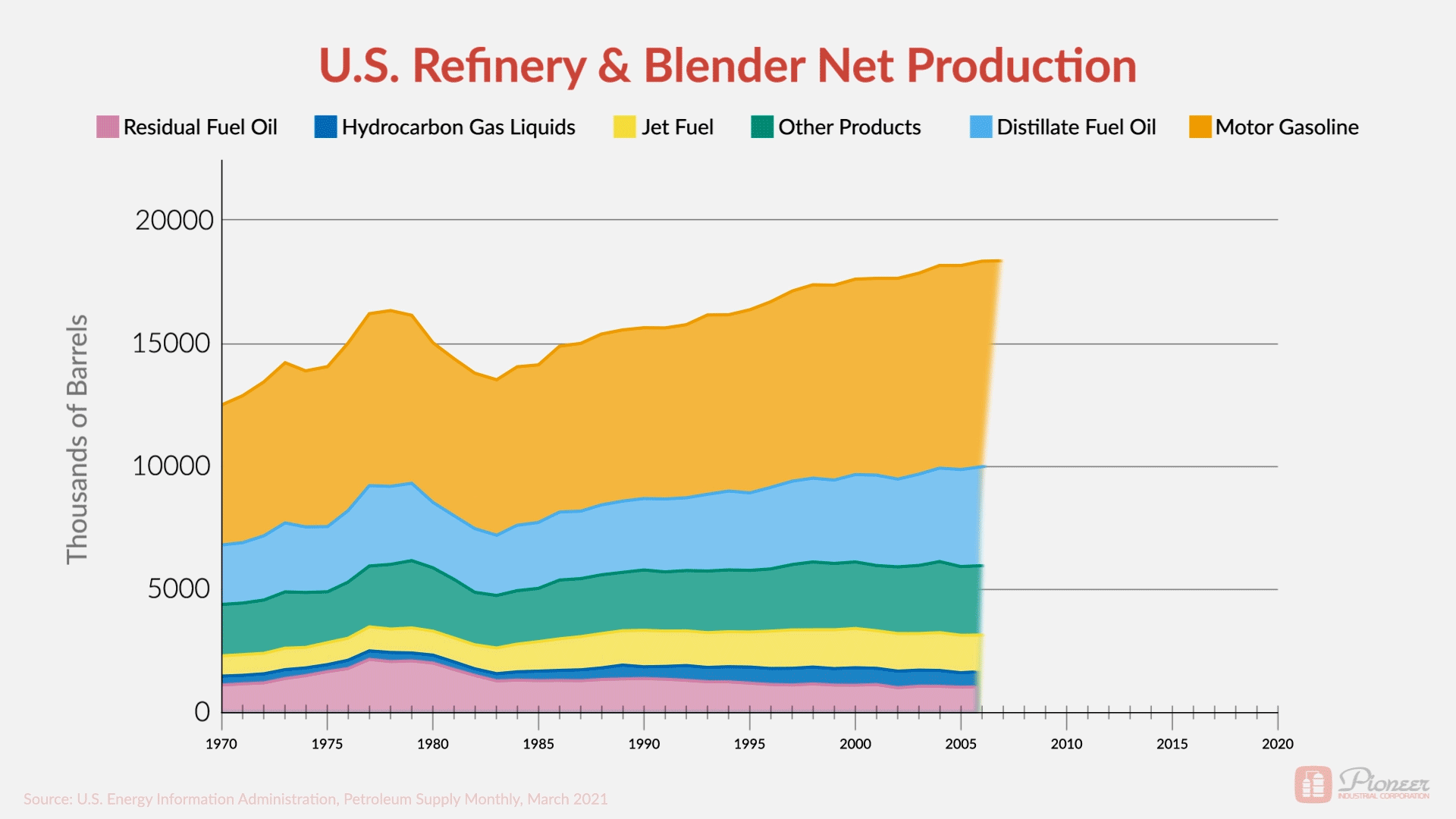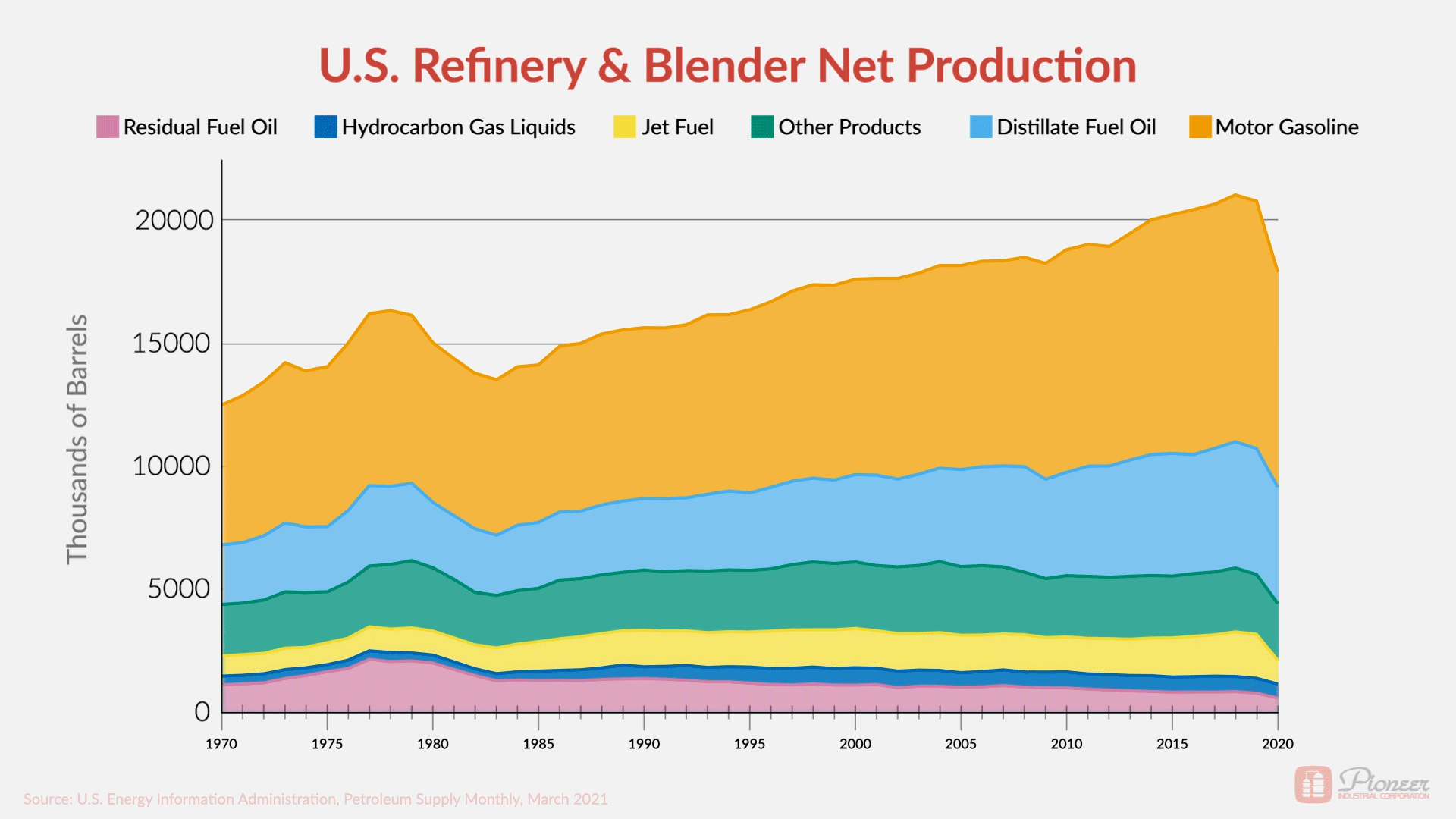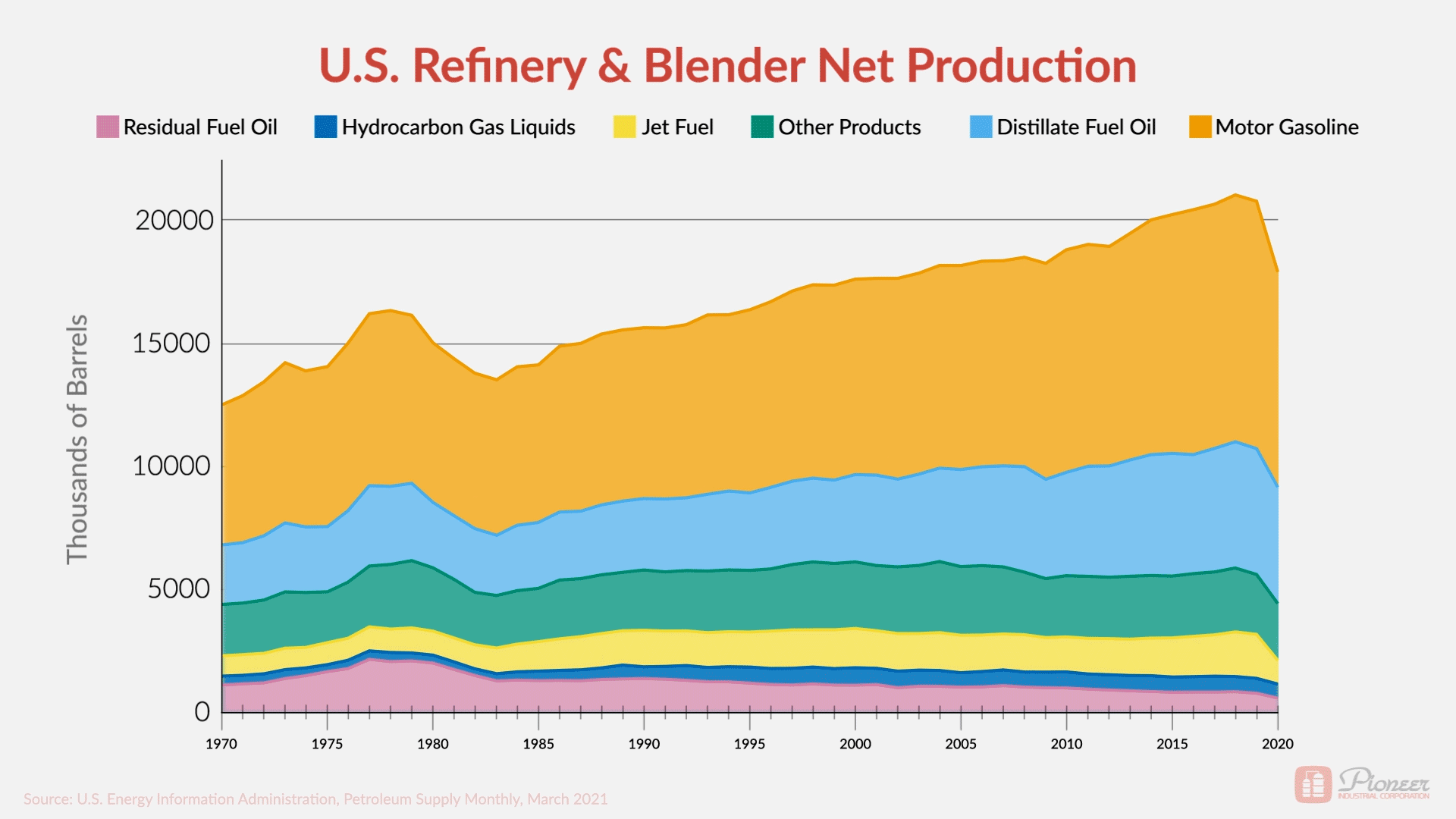
The graphic above illustrates the refined oil products we might expect most: gasoline, jet fuel, distillate, diesel, kerosene, and other fuels. However, the chemical by-products also have a significant impact on our lives. They go into the countless, essential products we use in our day-to-day lives.
Our Oil-Derived World
By now, you probably get the gist of it. Oil and gas are not solely a means of fueling our cars, heating our homes, or powering our infrastructure. It goes into so many components of everyday life that we would be left with some major problems if they suddenly disappeared tomorrow. Our modern world and quality of life owe a lot to the refining process.
Of course, there is always room for improvement in this industry. Sustainable alternatives, naturally sourced components, and biodegradable products can offer less pollution and a healthy earth. These advances are already underway. Nonetheless, even they are powered by the technology that oil-derived products have allowed us to accomplish.
 Copyright Independent Balkan News Agency, 2021
https://www.pioneerindustrial.com/wp-content/uploads/2021/11/Biogas-Plant.jpeg
1048
2000
Karrie Williams
https://www.pioneerindustrial.com/wp-content/uploads/2020/12/Pioneer-Logo-Color-min.png
Karrie Williams2021-11-17 21:25:392021-11-17 21:25:44Waste Burner Systems
Copyright Independent Balkan News Agency, 2021
https://www.pioneerindustrial.com/wp-content/uploads/2021/11/Biogas-Plant.jpeg
1048
2000
Karrie Williams
https://www.pioneerindustrial.com/wp-content/uploads/2020/12/Pioneer-Logo-Color-min.png
Karrie Williams2021-11-17 21:25:392021-11-17 21:25:44Waste Burner Systems
Harnessing Natural Fluid Flows – An Exploration of Alternative Energy Sources Industries
Environmental Safety, Renewable Energy

 Copyright 2019 © evon GmbH All rights reserved.
Copyright 2019 © evon GmbH All rights reserved.